Abstract
Introduction: Venetoclax (Ven) in combination with hypomethylating agents (HMAs) is approved to treat patients (pts) with newly diagnosed (ND) acute myeloid leukemia (AML) who are ineligible for intensive chemotherapy, based on the results from phase 1b (NCT02203773) and phase 3 (VIALE-A; NCT02993523) studies. This study aims to better understand pre- and post-VIALE-A real-world (rw) treatment (Tx) experience of Ven+HMA in routine clinical care.
Methods: This retrospective cohort study used the Flatiron Health electronic health record-derived, nationwide, de-identified database. Pts with ND AML, aged ≥18 years, who initiated Ven+HMA Tx ≤1 month from AML diagnosis between June 1, 2018, and March 31, 2021 (excluding HMA Tx prior to AML diagnosis) were included. The study cohort was stratified into pre- (before July 1, 2020) vs post-VIALE-A (July 1, 2020 onwards). Timing of bone marrow (BM) biopsy, rw complete response/complete response with partial hematologic recovery (rwCR/CRh; <5% BM blasts with a platelet count >50×109/L and absolute neutrophil count [ANC] >0.5×109/L), and rwCR/complete response with incomplete hematologic recovery (rwCR/CRi; <5% BM blasts with either ANC <1000×109/L or platelet <100×109/L) were measured. Tx schedule modifications were documented. Kaplan-Meier analyses summarized time to last administration, time to first BM biopsy, time to first response (<5% BM blasts), time to death, or censoring at the end of follow-up on January 31, 2022. Cox regression analyses with a time varying covariate (Tx schedule modification) were conducted to examine the association between Ven Tx schedule modifications and overall survival (OS).
Results: In total, 498 pts treated with Ven+HMA met the study criteria; most pts were treated in community practice (80%) vs academic settings (20%). Overall, 66% (330/498) of pts were pre-VIALE-A and 34% (168/498) were post-VIALE-A (median age was 76 and 75 years, secondary-AML was 33% and 27%, respectively). European LeukemiaNet 2017 classification: adverse, 42% vs 46%; intermediate, 22% vs 28%; favorable, 13% vs 9% for pre- vs post-VIALE-A cohorts, respectively.
The median time from diagnosis to Ven Tx initiation was 12 days for the pre-VIALE-A cohort and 14 days for the post-VIALE-A cohort. Median time to last administration was 4.34 months for the pre-VIALE-A cohort (median follow-up: 8.98 months) and 4.67 months for the post-VIALE-A cohort (median follow-up: 8.52 months). Fewer pts in the post- vs pre-VIALE-A cohort (13% [22/168] vs 19% [63/330]) discontinued ≤60 days after Tx initiation (p=0.10).
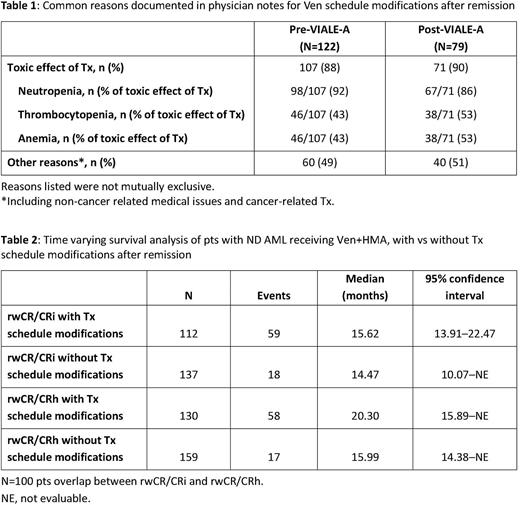
Overall, 65% (323/498) of pts with evaluable BM blast clearance were documented during follow-up and had low early mortality (death ≤60 days; <2% [<5/323]) vs 35% (175/498) of pts without an evaluable BM blast clearance who had higher early mortality (23% [40/175]). Amongst pts with BM blast clearance data available, more pts in the post- (61% [77/126]) vs pre-VIALE-A cohort (45% [101/225]) had their first biopsy by 28 ± 14 days after Tx initiation (proxy for Tx cycle 1). Earlier time to BM assessment after Ven Tx initiation in the post- vs pre-VIALE-A cohorts was associated with an earlier median time to first response (2.34 vs 5.14 months, respectively). More post- vs pre-VIALE-A pts with rwCR/CRi (n=45 vs n=93, respectively) had Ven schedule modifications after remission (days 0-30: 60% vs 39%; days 31-60: 36% vs 29%; anytime: 89% vs 74%). Most common reasons for Ven schedule modifications after remission, as documented in available physician notes (122 pre- vs 79 post-VIALE-A) were: toxic effect of Tx (88% vs 90%) and other reasons e.g. non-cancer related medical issues and cancer-related Tx (49% vs 51%; Table 1). Time-varying survival analysis showed that Ven Tx schedule modifications after remission were associated with longer median OS (Table 2).
Conclusions: This study observed an evolution of earlier BM biopsy and an improvement in Ven Tx schedule modification in pts with ND AML who received Ven+HMA from pre- to post-VIALE-A, in a predominantly community setting in the US. Cytopenia was noted as a common reason for Ven Tx schedule modifications. Ven Tx schedule modifications after remission were associated with better median OS. Opportunities remain to further improve the number of pts with earlier BM assessment to determine Ven Tx schedule modifications and provide the best chance for optimal Tx outcomes for pts.
Disclosures
Vachhani:Incyte: Speakers Bureau; Blueprint Medicines, Incyte, AbbVie, CTI BioPharma Corp, Novartis, Amgen, Pfizer, Genentech, Stemline: Consultancy. Ma:Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company. Xu:F. Hoffmann La-Roche: Current Employment, Current equity holder in private company. Montez:Genentech/Roche: Current Employment, Current equity holder in publicly-traded company; Roche: Current equity holder in publicly-traded company. Worth:University of Alabama at Birmingham: Current Employment; JADPRO Expert Conversations in CML: Honoraria; Blueprint Medicines, CTI BioPharma Corp: Membership on an entity's Board of Directors or advisory committees. Cheng:AbbVie: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Werner:AbbVie: Current Employment, Current equity holder in private company. Abbas:Tennessee Oncology: Current Employment; AbbVie, Bristol Myers: Consultancy, Speakers Bureau; Incyte, Morphosys: Speakers Bureau. Donnellan:Janssen, Merck, Pfizer, Amgen, Gilead: Consultancy; Merck, Pfizer, Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal